More Temperature and Line Strength
Different elements absorb light, and thereby produce absorption lines, at different temperatures. You found in previous exercises that a star needs just the right temperature for the Hα transition to occur.
Because absorption lines require that electrons start at a certain energy level, different absorption lines are prominent at different temperatures. The graph below shows the relative line strength for a given element versus temperature.
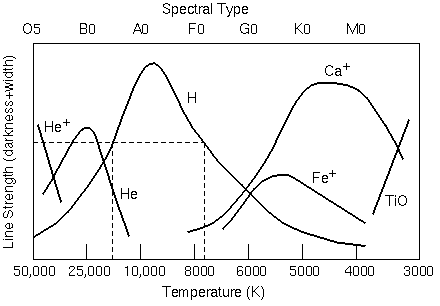
Line strength versus temperature for several elements whose absorption lines are commonly seen in stellar spectra
Look back at your answer to Question 12 – the spectral class with the strongest hydrogen absorption. Does that answer make sense when you compare it with what you see in the graph?
Research Challenge 1. So far in this project, you have found the spectral class with the right temperature for Hα absorption. Do the same for neutral sodium (Na). Look at the stellar spectra again, but this time look specifically at the strength of the neutral sodium line* at 5890 Ångstroms. Which type of star shows the strongest sodium absorption? It may help to look through the spectra some other stars, and the Plate Browser will help you do that.
* The neutral sodium line is actually two very closely spaced lines at 5890 and 5896 Ångstroms.
Another Way to Classify
We have used two different methods to classify stars – the hydrogen lines (Hα, Hβ, Hγ, Hδ) and the continuum peak wavelength. But professional astronomers usually use a slightly different method. They look for the presence (or absence) of several spectral lines to classify stars.
The table below lists some of the spectral lines that they look for, and the lines’ approximate locations in the electromagnetic spectrum.
| Spectral Lines | Wavelengths (Ångstroms) |
|---|---|
| Hydrogen: Ha, Hb, Hg | 6560, 4860, 4340 |
| Ionized Calcium | 3970, 3930 (labeled H and K) 8490, 8540, 8660 (labeled Ca II) |
| Titanium Oxide | lots of lines from 4900 – 5200, 5400 – 5700, 6200 – 6300, 6700 – 6900 |
| G Band | 4220 |
| Sodium | 5890 |
| Helium (neutral – labeled He) | 4200 |
| Helium (ionized – labeled He+) | 4400 |
Once they have found the spectral lines they need, they classify the stars based on what spectral lines are present. Here are the lines they look for to identify what spectral class a star belongs to:
| Spectral Type | Spectral Lines |
|---|---|
| O | Ionized helium |
| B | Helium, some hydrogen |
| A | Strong hydrogen, some ionized metals such as calcium |
| F | Hydrogen, ionized calcium, iron |
| G | Neutral and ionized metals, especially calcium; methine |
| K | Neutral metals, sodium |
| M | Strong titanium oxide (TiO), very strong sodium |
Research Challenge 2. Use the information in the tables above to classify the following stars (click the links to open the Quick Look tool):
If you would like to classify more stars, you can find many more with the SkyServer Plate Browser.
